|
Water is not only the most common substance on Earth, because
it is also one of the most unusual. No other substance can do
all the things that water can do, making it an exception to many
of nature's rules, because of its unusual properties. Water consists
of tiny particles called molecules; each molecule consists of even
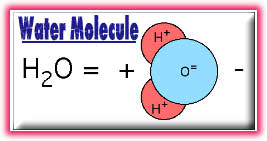
smaller particles called atoms. Water molecules consist of hydrogen
and oxygen atoms, which are gases on their own. The atoms in a water
molecule are arranged with the two H-O bonds at an angle of about
105�, rather than on directly opposite sides of the oxygen atom.
The two hydrogen atoms and one oxygen atom combine to form the
chemical compound H2O-- water.
Water can be a solid, liquid, or gas. No other substance appears
in these three forms within Earth's normal range of temperature.
- Ice
- When water is cooled, it contracts only until its
temperature reaches 4�C. Water expands when it becomes
colder than that. For this reason, when ice forms at
0�C, it floats on liquid water.
- Liquid
- Water is a liquid at temperatures found in most places on
Earth. Water is a liquid between 0�C and 100�C, its freezing
and boiling points.
- Vapor
- Uncovered water will gradually disappear over a few days, due
to the constant motion of water molecules. Those at the surface
break free of those below and enter the air as vapor. When water
is heated, the molecules move faster, so steam is created.
Water also has the greatest heat capacity of any other substance except
ammonia. A way to illustrate this is to imagine a pound of water, gold,
and iron-- all at -273.15�C. If all three were heated and each absorbed the
same amount of energy, when the gold would melt at 1102�C, the ice would
be at -184�C. When the iron would begin to melt at 1299�C, the ice would
finally have reached 0�C.
In addition, water has capillarity, a dissolving ability, and extremely
high surface tension.
|
|

